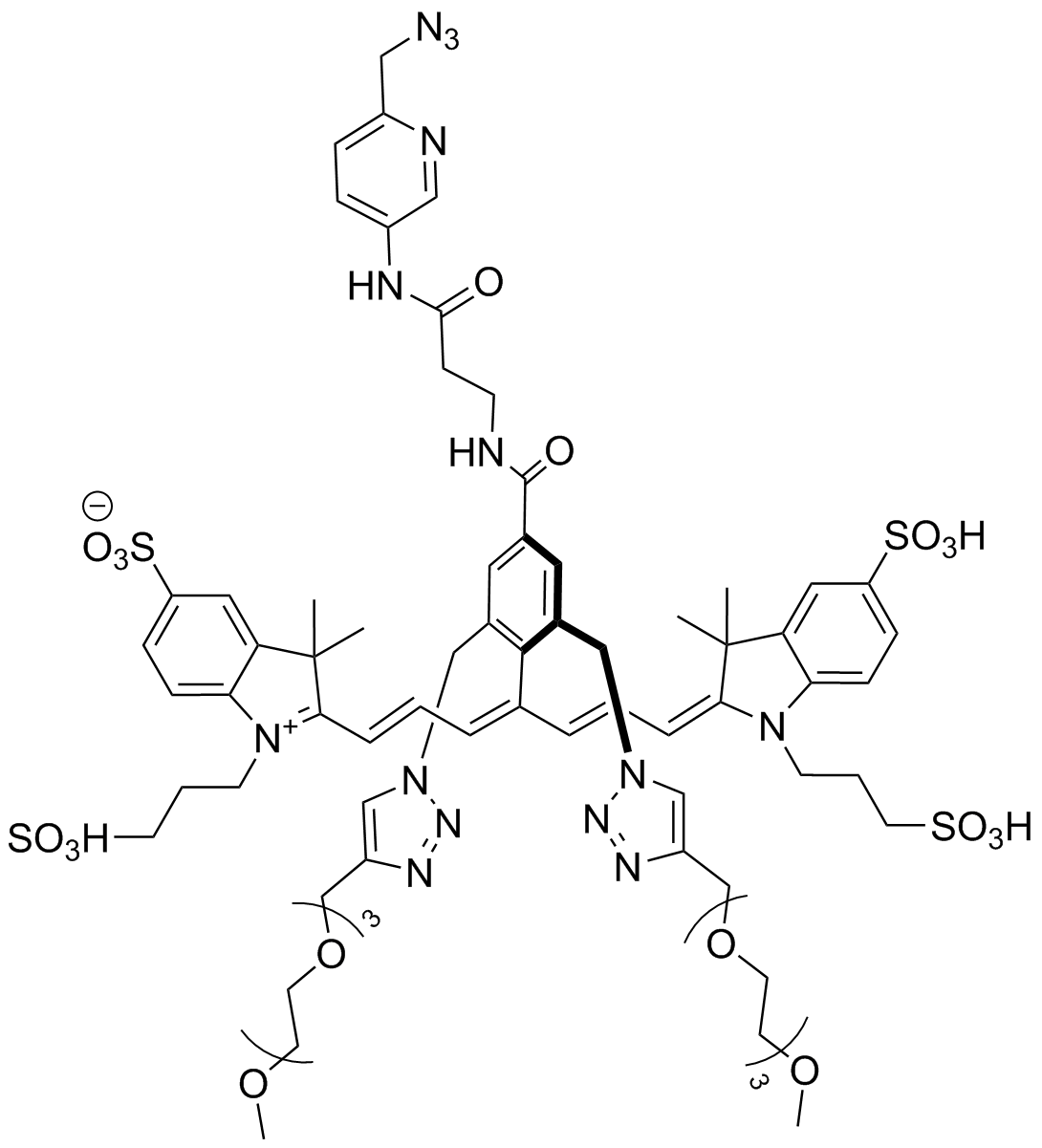
AZDye 800 Picolyl Azide
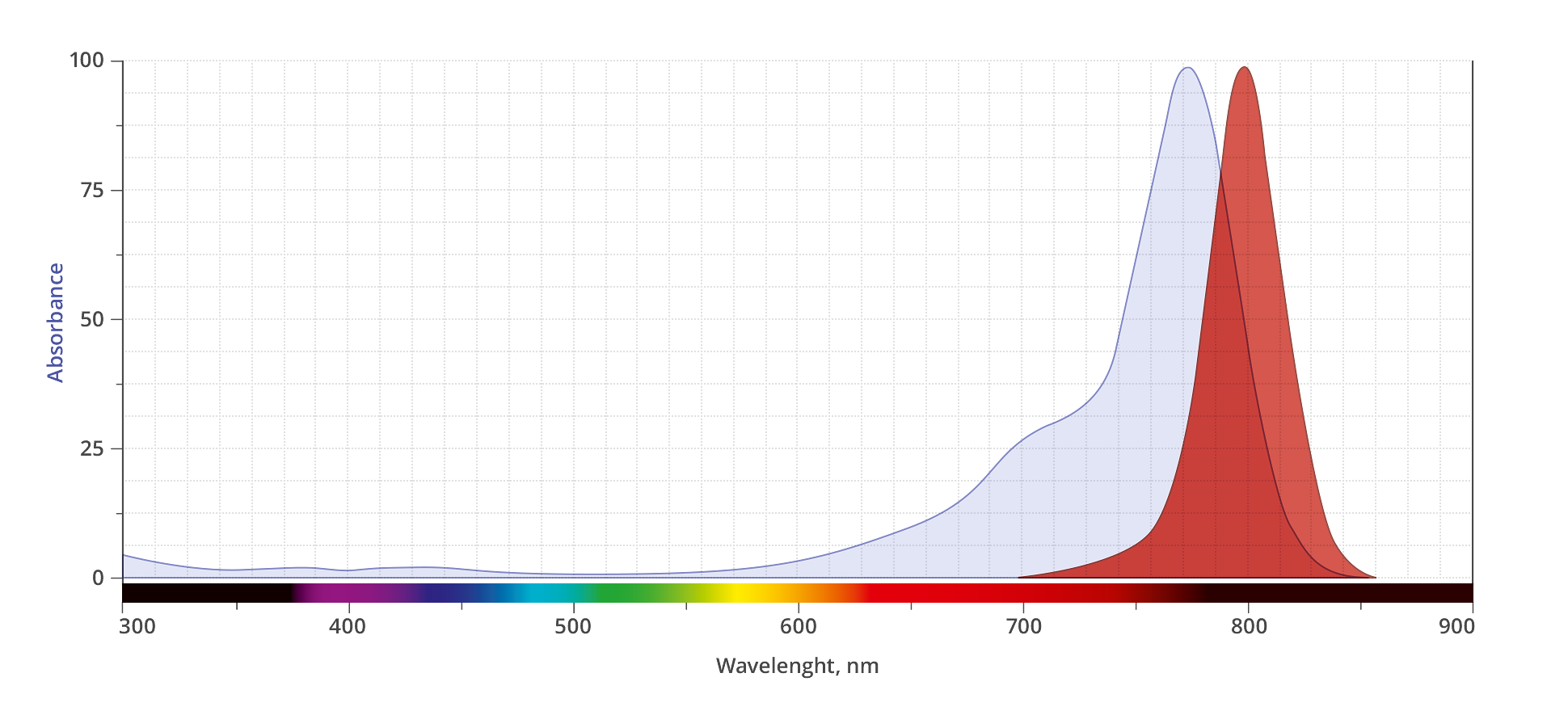
Abs/Em Maxima: 775/7791 nm
Extinction Coefficient: 206,000
Spectrally Similar Dyes: IRDye? 800CW, CF? 800, DyLight? 800
AZDye? 800 Picolyl Azide is an advanced fluorescent probe that incorporates a copper-chelating motif to raise the effective concentration of Cu(I) at the reaction site to boost the efficiency of the CuAAC reaction, resulting in a faster and more biocompatible CuAAC labeling. Up to 40-fold increase of signal intensity, compared to conventional azides, was reported (see Selected References).
AZDye? 800 is a conceptually new class of sterically shielded NIR cyanine heptamethine dye. This dye contains two shielding PEG arms directly over both faces of the heptamethine fluorochrome blocking any undesired bimolecular association processes and thus dramatically enhances the fluorescence brightness. Its excitation ideally suited for the 800nm channel of imaging systems. AZDye? 800 is spectrally is almost identical to IRDye 800CW and DyLight 800.
Cyanine heptamethine dyes are well-known NIR fluorophores, with emission wavelengths >740 nm, and often are reagents of choice for labeling antibodies. Two of the most popular commercial NIR cyanine heptamethine dyes for antibody conjugation are IRDye 800CW and DyLight 800. While these NIR dyes are undoubtedly useful for many types of immunofluorescence technologies, the resulting NIR dye-labeled antibodies sometimes exhibit performance limitations due to three inherent fluorophore concerns. (1) A meso-OAryl group connected directly to the heptamethine fluorochrome group is susceptible to nucleophilic displacement by biological amines and thiols1, 2 resulting in a diminished chemical stability of the dye-antibody conjugates during synthesis, storage, or the time-course of an imaging experiment. In addition, the electron-donating meso-OAryl group promotes high fluorochrome reactivity with electrophilic singlet oxygen, resulting in relatively poor dye photostability.3,4 (2) Both dyes are flat molecules with a hydrophobic core and a polyanionic charge periphery. Thus, the chemical conversion of a small polar, cationic lysine residue on the antibody surface to a large hydrophobic, polyanionic dye derivative has the potential to produce substantial changes in antibody folding and physiochemical properties, leading to lower antibody stability and decreased target specificity.4-8 (3) When activated versions of these hydrophobic dyes are conjugated to an antibody surface, they tend to attach at the proximal lysine sites as stacked face-to-face dimers, which produces a diagnostic H-dimer peak in the absorbance spectra that is nonfluorescent.8,9 Moreover, the close stacking of proximal conjugated IRDye 800CW or DyLight800 on an antibody surface amplifies the potential for a deleterious effect on antibody targeting because of a localized patch of polyanionic charge and hydrophobicity.
In order to address these drawbacks of commercially NIR cyanine a conceptually new class of sterically shielded NIR dyes was developed in Dr. Bradley D. Smith laboratory.10, 11 These dyes contain two shielding PEG arms directly over both faces of the heptamethine fluorochrome blocking any undesired bimolecular association processes and thus enhance the fluorescence brightness. A recently published antibody labeling study clearly demonstrated 1-2 order of magnitude increase in brightness compared to commercially available NIR dyes as a result of almost complete prevention of stacking of multiple fluorophores appended to the antibody surface.10, 11
These sterically shielded NIR dyes with greatly improved chemical and photochemical stability and substantially enhanced brightness will enable researchers to greatly improve various types of indirect NIR immunofluorescence imaging and diagnostics applications that require high sensitivity and also develop new photonintense techniques that require high photostability.
MB 800Z is a zwiteroinic, charge-balanced dye with an equal number of anionic sulfonate and cationic ammonium residues, a structural feature that is known to reduce interactions with off -target biological surfaces.12,13
Selected References:
1. Van Leeuwen, F. W. B., et al. (2016). Synthesis and systematic evaluation of symmetric sulfonated centrally Csingle bondC bonded cyanine near-infrared dyes for protein labelling. Elsevier, Dyes and Pigments, 132, 7-19. [ScienceDirect]
2. Wheat, T. E., et al. (2002). IRDye78 conjugates for near-infrared fluorescence imaging Mol Imaging, 1 (4), 354-64. [PubMed]
3. Clarke, D. T., et al. (2012). Multicolour Single Molecule Imaging in Cells with Near Infra-Red Dyes. PNAS, No. e36265. [PLoS One]
4. Robinson, C. M., et al. (2019). A Nonaggregating Heptamethine Cyanine for Building Brighter Labeled Biomolecules. ACS Chem. Biol, 14, 934?940. [ACS Publications]
5. Gibbs, S., et al. (2013). Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat Biotechnol, 31, 148-153. [Nature Biotechnology]
6. Hartman, Y., et al. (2015). Fluorescence-guided resection of experimental malignant glioma using cetuximab-IRDye 800CW. Br J Neurosurg., 29, 850–858. [PubMed]
7. Vereb, G., et al. (2018). The Effect of Fluorophore Conjugation on Antibody Affinity and the Photophysical Properties of Dyes. Biophys. J., 114, 688-700. [PubMed]
8. Harms, G. S., et al. (2000). Anomalous Fluorescence Enhancement of Cy3 and Cy3.5 versus Anomalous Fluorescence Loss of Cy5 and Cy7 upon Covalent Linking to IgG and Noncovalent Binding to Avidin. Bioconjugate Chem., 11, 696?704. [ACS Publications]
9. Hamann, F. M., et al. (2011). Suitable Labels for Molecular Imaging – Influence of Dye Structure and Hydrophilicity on the Spectroscopic Properties of IgG Conjugates. Bioconjugate Chem., 22 (7), 1298–1308. [ACS Publications]
10. Smith, B. D, et al. (2020). Sterically Shielded Heptamethine Cyanine Dyes for Bioconjugation and High Performance Near‐Infrared Fluorescence Imaging. Angew. Chem. Int. Ed., 59, 12154–12161. [PubMed]
11. Smith, B. D, et al. (2021). High-Performance Near-Infrared Fluorescent Secondary Antibodies for Immunofluorescence. Analytical Chemistry, 93 (7), 3643-3651. [PubMed]
12. Schnermann, M. J., et al. (2016). Role of Fluorophore Charge on the In Vivo Optical Imaging Properties of Near-Infrared Cyanine Dye/Monoclonal Antibody Conjugates . Bioconjugate Chem., 27 (2), 404-13. [PubMed]
13. Ramsey, J. D., et al. (2020). Interactions between Biomolecules and Zwitterionic Moieties: A Review. Biomacromolecules, 21 (7), 2557-2573. [PubMed]
1. Jiang, H., et al. (2014). Monitoring Dynamic Glycosylation in Vivo Using Supersensitive Click Chemistry. Bioconjugate Chem.,, 25, 698-706. [PubMed]
2. Uttamapinant, C., et al. (2012). Fast, Cell-Compatible Click Chemistry with Copper-Chelating Azides for Biomolecular Labeling. Angew. Chem. Int. Ed,., 51, 5852-56. [PubMed]
3. Gaebler, A.,et al. (2016). A highly sensitive protocol for microscopy of alkyne lipids and fluorescently tagged or immunostained proteins. J. Lipid. Res., 57, 1934-47. [PubMed]
Abs/Em Maxima: 346/445 nmExtinction Coefficient: 19,000Spectrally Similar Dyes: Alexa Fluor? 350, CF? 350, DyLight 350, AMCAAZDye? 350 is a blue-fluorescent azide-activated probe that reacts with terminal alkynes via a copper-catalyzed click react…
Abs/Em Maxima: 402/424 nmExtinction Coefficient: 35,000Flow Cytometry Laser Line: 405 nmMicroscopy Laser Line: 405 nmSpectrally Similar Dyes: Alexa Fluor? 405, CF? 405, Cascade Blue?, DyLight? 405AZDye? 405 Azide is a water-soluble, pH-insensiti…
Abs/Em Maxima: 346/445 nmExtinction Coefficient: 19,000Spectrally Similar Dyes: Alexa Fluor? 350, CF? 350, DyLight 350, AMCAAZDye? 350 Alkyne (Alexa Fluor? 350 Alkyne equivalent) is a blue-fluorescent, alkyne-activated probe that reacts with azid…
Abs/Em Maxima: 430/537 nmExtinction Coefficient: 15,000Spectrally Similar Dyes: Alexa Fluor? 430, CF? 430AZDye? 430 Azide is a water-soluble, green-fluorescent azide-activated probe that reacts with terminal alkynes via a copper-catalyzed click re…
Abs/Em Maxima: 346/445 nmExtinction Coefficient: 19,000Spectrally Similar Dyes: Alexa Fluor? 350, CF? 350, DyLight 350, AMCAAZDye? 350 DBCO reacts with azides via a copper-free “click chemistry” reaction to form a stable triazole and does not re…
Abs/Em Maxima: 402/424 nmExtinction Coefficient: 35,000Flow Cytometry Laser Line: 405 nmMicroscopy Laser Line: 405 nmSpectrally Similar Dyes: Alexa Fluor? 405, CF? 405, Cascade Blue?, DyLight? 405AZDye? 405 Alkyne reacts with azides via a copper…
Abs/Em Maxima: 494/517 nmExtinction Coefficient: 73.000Flow Cytometry Laser Line: 488 nmMicroscopy Laser Line: 488 nmSpectrally Similar Dyes: Fluorescein, Alexa Fluor? 488, CF? 488A, DyLight? 488, Atto? 488AZDye? 488 Azide (Alexa Fluor? 488 Azi…
Abs/Em Maxima: 402/424 nmExtinction Coefficient: 35,000Flow Cytometry Laser Line: 405 nmMicroscopy Laser Line: 405 nmSpectrally Similar Dyes: Alexa Fluor? 405, CF? 405, Cascade Blue?, DyLight? 405ZDye? 405 DBCO reacts with azides via a copper-fr…
