Azide Plus 試劑
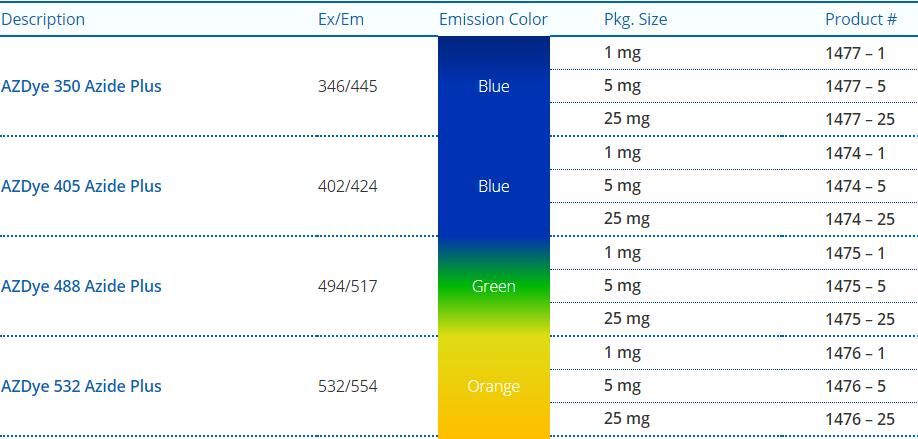
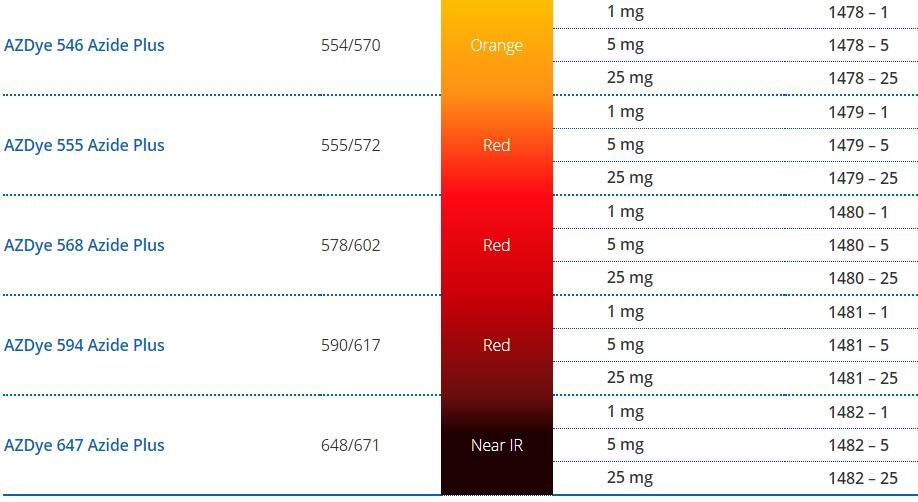
Azide Plus 試劑詳情介紹一覽表
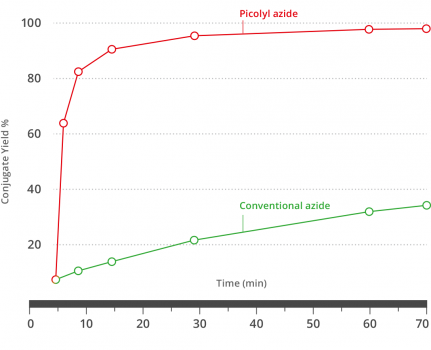
Figure 1). Kinetic comparison of chelating azide and
non-chelating conventional azide
Recent advances in the design of copper-chelating ligands, such as
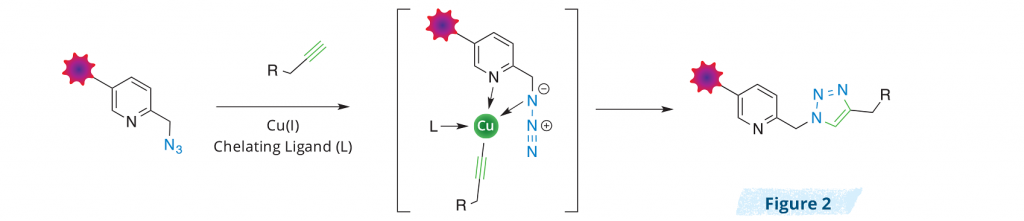
 THPTA or BTTAA that stabilize the Cu(I) oxidation state in aqueous solution, improve the kinetics of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction and greatly increase the sensitivity of alkyne detection. Copper-chelating ligands have also been shown to increase the biocompatibility of the CuAAC reaction by preventing the copper ions from causing biological damage1. The next step in improving the CuAAC reaction was the development of copper-chelating azides as more reactive substrates. Since it is speculated that the Cu(I)-azide association is the rate-determining step in the CuAAC catalytic cycle2, the introduction of a copper-chelating moiety at the azide reporter molecule allows for a dramatic raise of the effective Cu(I) concentration at the reaction site, enhancing the weakest link in the reaction rate acceleration (Figure 2). It has been proposed that the high reactivity of chelating azides comes from the rapid copper-azido group interaction which occurs prior to Cu(I) acetylide formation, and this renders the deprotonation of alkyne in the rate-determining step3. This concept was successfully exploited to perform CuAAC reactions using pyridine-based copper-chelating azides (picolyl azides) as substrates4-6. Nevertheless, the copper-chelating motif of picolyl azide molecules is not complete, requiring the presence of a copper chelator (e.g. THPTA) to achieve significant improvement in the kinetics of the CuAAC reaction3, 4.
THPTA or BTTAA that stabilize the Cu(I) oxidation state in aqueous solution, improve the kinetics of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction and greatly increase the sensitivity of alkyne detection. Copper-chelating ligands have also been shown to increase the biocompatibility of the CuAAC reaction by preventing the copper ions from causing biological damage1. The next step in improving the CuAAC reaction was the development of copper-chelating azides as more reactive substrates. Since it is speculated that the Cu(I)-azide association is the rate-determining step in the CuAAC catalytic cycle2, the introduction of a copper-chelating moiety at the azide reporter molecule allows for a dramatic raise of the effective Cu(I) concentration at the reaction site, enhancing the weakest link in the reaction rate acceleration (Figure 2). It has been proposed that the high reactivity of chelating azides comes from the rapid copper-azido group interaction which occurs prior to Cu(I) acetylide formation, and this renders the deprotonation of alkyne in the rate-determining step3. This concept was successfully exploited to perform CuAAC reactions using pyridine-based copper-chelating azides (picolyl azides) as substrates4-6. Nevertheless, the copper-chelating motif of picolyl azide molecules is not complete, requiring the presence of a copper chelator (e.g. THPTA) to achieve significant improvement in the kinetics of the CuAAC reaction3, 4.
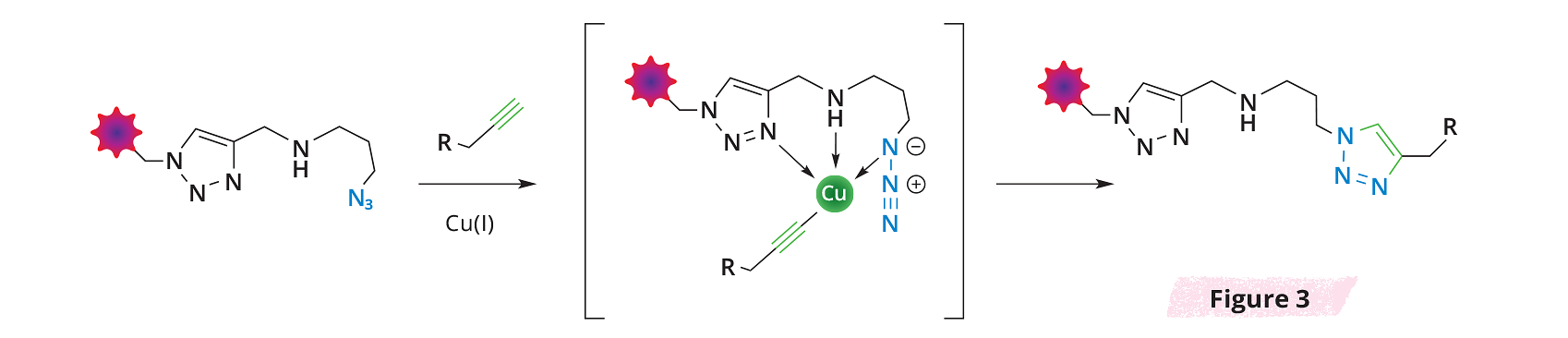
In efforts to improve the performance of the CuAAC reaction in complex media, Click Chemistry Tools developed new chelating azides with a complete copper-chelating system in their structure, termed “Azides Plus” (Figure 3). These azides are capable of forming strong, active copper complexes and are therefore considered both reactant and catalyst in the CuAAC reaction. Using these types of azides, the CuAAC reaction becomes a bimolecular reaction and displays much faster kinetics compared to the CuAAC reaction performed with conventional azides.
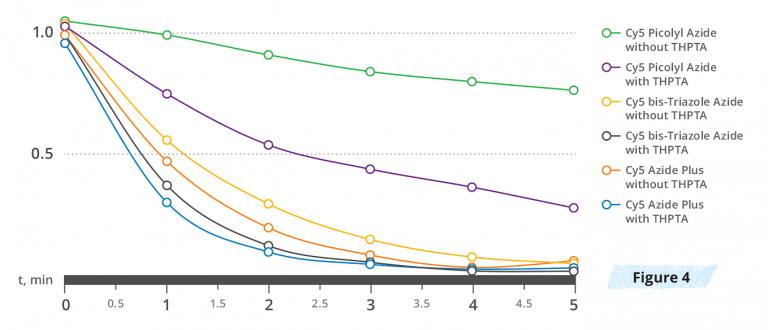
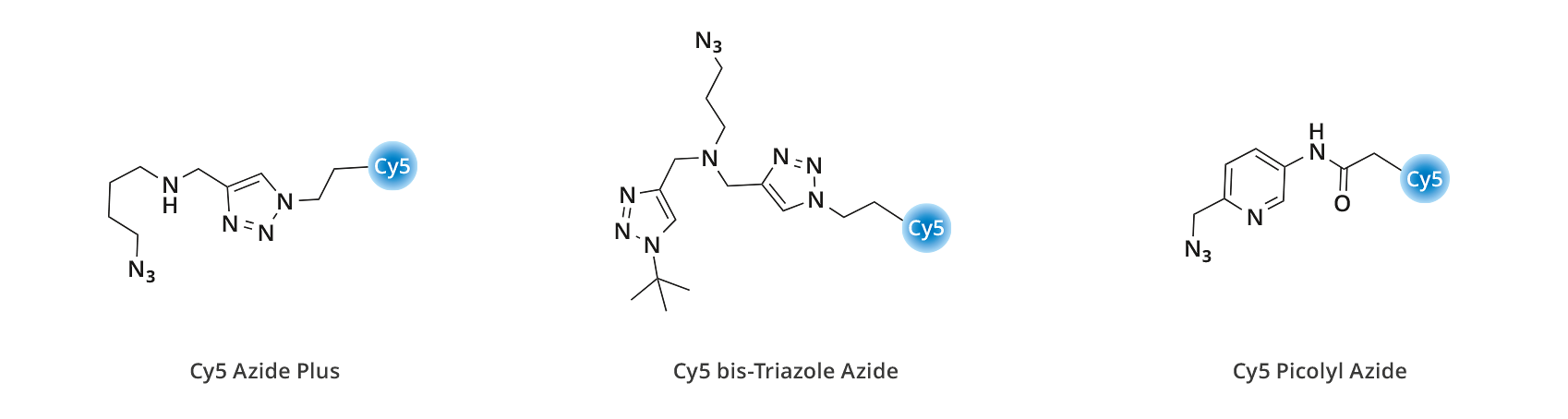
Comparative kinetic measurements for the CuAAC reaction (Figure 4) were performed using an agarose-alkyne resin labeling experiment (3.0 uM CuSO4, with (6.0 uM) or without THPTA ligand) using Cy5 Azide Plus, Cy5 Picolyl Azide, and Cy5 bis-Triazole Azide – the fastest copper-chelating azide that has been reported to date7. As expected, the picolyl azide containing the incomplete copper-chelating motif displays relatively slow reactivity, in particular without the presence of THPTA. The kinetic data shows that completing a copper-chelating moiety greatly enhances reactivity, and importantly does not require the presence of copper-chelating ligands. Interestingly, the copper-chelating azides developed by Click Chemistry Tools display almost identical reactivity in the CuAAC reaction compared to the most reactive copper-chelating azide reported up to now7, bis-triazole azide.
The new copper chelating azides allow the formation of azide copper complexes that react almost instantaneously with alkynes under diluted conditions. This unprecedented reactivity in the CuAAC reaction is of special value for the detection of low abundance targets, improving biocompatibility, and any other application where greatly improved S/N ratio is highly desired.
Selected References:
1. Hong, V.,et al.(2010). Labeling Live Cells by Copper-Catalyzed Alkyne?Azide Click Chemistry.Bioconjugate Chem.,21: 1912–6.
2. Rodionov, V.O.,et al.(2007). Ligand-accelerated Cu-catalyzed azide-alkyne cycloaddition: A mechanistic report.J. Am. Chem. Soc.,129: 12705–12.
3. Presolski, S.I.,et al.(2010). Tailored ligand acceleration of the cu-catalyzed azide-alkyne cycloaddition reaction: Practical and mechanistic implications.J. Am. Chem. Soc.,132: 14570–6.
4. Kuang, G.-C.,et al.(2011). Experimental investigation on the mechanism of chelation-assisted, copper (ii) acetate-accelerated azide-alkyne cycloaddition.J. Am. Chem. Soc.,133: 13984–4001.
5. Jiang, H., et al. (2014). Monitoring Dynamic Glycosylation in Vivo Using Supersensitive Click Chemistry. Bioconjugate Chem., 25: 698-706.
6. Uttamapinant, C.,et al.(2012). Fast, Cell-Compatible Click Chemistry with Copper-Chelating Azides for Biomolecular Labeling. Angew. Chem. Int. Ed,.51: 5852.
7. Gaebler A.,et al.(2016). A highly sensitive protocol for microscopy of alkyne lipids and fluorescently tagged or immunostained proteins. J. Lipid. Res,.57: 1934-47.
8. Bevilacqua, V.,et al.(2014). Copper-Chelating Azides for Efficient Click Conjugation Reactions in Complex Media. Angew. Chem. Int. Ed,.53: 5872-6.
Ordering information